Chronic Prostatitis: Over 70% Bacterial
Copyright © 2016 Bacterial Prostatitis Collective. May be reproduced for non-commercial purposes.
Abstract
BACKGROUND
Prevailing medical opinion holds that only 5-10% of chronic prostatitis is due to bacteria.
If the usual prescriptions of oral antibiotics
cured long-standing cases of chronic prostatitis, everyone would be satisfied
and that would be the end of it. That is not the case. The embrace of the
non-bacterial etiology has been motivated by the failure of antibiotics.
However, some experienced physicians believe most prostatitis is bacterial, and
the controversy persists.
METHODS
We undertook a review of the literature in order to
reconcile these divergent views, with particular attention to studies cited by
the proponents of non-bacterial prostatitis.
RESULTS
When prostatitis is treated promptly at the onset
of symptoms, a large majority of prostatitis patients are cured. Long
standing cases are resistant. Why? Because given time, bacteria in the
prostate form biofilms, which render bacteria resistant to ordinary
concentrations of antibiotics. Prompt antibiotic treatment upon emergence of
symptoms is the key to preventing chronic prostatitis. The
belief that prostatitis is almost entirely nonbacterial, and the resulting
failure to treat it promptly, is largely responsible for the widespread
prevalence of intractable chronic prostatitis.
Some studies find a low incidence
of bacteria in prostatitis patients' prostates. In these studies a single
massage was used to expel bacteria from the prostate. In order to reliably
expel bacteria from the prostate, several massages are required. The low
incidence of bacteria found by single-massage studies is a result of an inadequate
methodology. Studies that utilize prostate biopsy find that over 70% of
prostatitis patients have bacteria in their prostates.
Antibiotics suppress the
activity of biofilm bacteria, which results in the
temporary relief of prostatitis symptoms for the duration of the treatment.
Non-bacterial prostatitis advocates believe such relief is due to an
anti-inflammatory effect other than suppression of bacteria. Fluoroquinolones are the mainstay of prostatitis
treatment. They exhibit abundant pro-inflammatory effects. In some
cases these pro-inflammatory effects have been a key component of fluoroquinolone effectiveness. A secondary
"anti-inflammatory" effect cannot be attributed to these
antibiotics.
Asymptomatic
prostate inflammation is even more common than symptomatic prostatitis. Why
are some cases symptomatic and others not? The bacteria of asymptomatic
prostatitis are different from those of symptomatic prostatitis - among
them are intracellular parasites that cause diffuse microscopic
inflammation. These only invade a few cells at a time and form dispersed
colonies. Chronic prostatitis, symptomatic or not, is associated with elevated
rates of prostate cancer and should be treated even if asymptomatic.
The wide prevalence of bacteria in healthy
prostates has been cited as a reason to doubt bacteria cause prostatitis.
However, some important pathogens only cause disease in a minority of hosts. At
most 10% of latent tuberculosis results in disease, and similarly with H.
pylori. The failure of a species of bacteria to cause disease in most hosts
does not exonerate it from being a serious pathogen.
How about the role of muscle tension and relaxation therapy? Prostatitis is very stressful and most patients get some benefit from such treatments. However, the population of patients who are cured by this treatment is distinct from the bacterial prostatitis population.
CONCLUSION
Over 70% of prostatitis is bacterial. Vigilance
and prompt antibiotic treatment is needed to reduce the current high prevalence
of chronic prostatitis, with its attendant personal and societal costs. In the event
of antibiotic failure there are alternative treatments that can mitigate symptoms
Abbreviations
BPH benign prostatic hyperplasia
CBP chronic bacterial prostatitis
CP/CPPS chronic prostatitis / chronic pelvic pain syndrome
EPS expressed prostatic secretions (fluid obtained from the prostate via prostate massage)
FQs fluoroquinolones
MPS myofascial pain syndrome
NIH National Institutes of Health
NIH-CPSI National Institutes of Health chronic prostatitis symptom index
PCR polymerase chain reaction (for amplification of DNA)
TURP trans-urethral resection of the prostate
UTI urinary tract infection
VB3 Voided Bladder 3 (urine collected after prostate massage)
WBCs/HPF white blood cells per high power field
Pro-inflammatory cytokines:
GM-CSF granulocyte macrophage colony-stimulating factor
IFN-γ interferon gamma
IL-1 interleukin-1
OPN osteopontin
TNF tumor necrosis factor
----------------------
Size of the problem
The prevalence of chronic prostatitis is reported to range from 4% to 13% of the adult male population.[1], [2], [3] The impact on quality of life is severe: “The quality of life of men with chronic prostatitis is dismal and significantly below that of patients with BPH, as well as most patients with prostate cancer,”[4] and “... scores were lower [worse] than those observed in the most severe subgroups of patients with congestive heart failure and diabetes,”[5] Regarding treatment, “Available treatment, especially for the chronic prostatitis syndromes, is poor, with no standard therapy producing significant cure rates.”[6] So it behooves us to understand the cause of this condition.
The controversy
In the
Examining the
arguments against bacteria as a cause of prostatitis
Argument 1: Most
prostatitis patients do not have inflammation related bacteria in their
prostates
The statement that only 5-10% of prostatitis is bacterial is found frequently in the literature. The primary reference supporting this contention is a large study by Weidner.[7] It is cited, for example, in "Chronic Prostatitis: An Infectious Disease?", by Nickel,[8] which lays out the arguments for and against bacterial prostatitis, and a recent medical text by Shoskes.[9]
Let us examine the Weidner study. Over 1400 prostatitis patients were studied over a period of 12 years. The authors state that only 5-10% “were diagnosed with bacterial prostatitis”, primarily due to E. coli or Enterococcus faecalis. But, reading further, we find "Ureaplasma-associated prostatitis" was detected in an additional 4-11.7% of patients but in none of the controls. The authors devote considerable discussion to the methodology of making this determination but do not include these cases in their oft-cited bacterial prostatitis total. Additionally, Chlamydia was cultured in 14.9% of patients vs. 5% of controls. This was not included in the 5-10% bacterial prostatitis total either. So, taking this paper at face value, about 30% of patients likely had bacterial prostatitis.
More importantly, there are methodological problems that
understate the true incidence of bacteria detected in the prostate. Weidner
does not state how much time they allowed for bacteria to propagate in culture.
Two days is standard practice. Allowing five days, instead of two, will
increase the number of positive EPS samples by 7.5%.[10]
But the foregoing is not the main methodological problem
with this study. The main problem is that
in most cases only one prostate massage was performed to expel EPS from the
prostate. Several massages spread over a number of days are required to
reliably expel bacteria from the prostate:
For bacteria and associated inflammatory markers to exit the prostate, they must first journey from the acini, through the prostatic ducts, and then into the urethra. It may take several days for this process to complete. A look at the accompanying micrograph shows why this is the case – the ducts leading out of the acini are long and narrow.
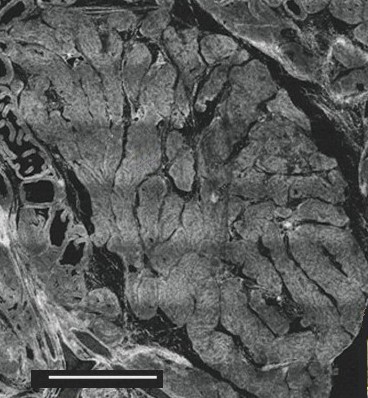
Figure 1
Cross-section of prostate gland showing prostatic
acini on left and ducts in center.
Scale bar: 1.5 mm [11]
According to work by Feliciano,[12] only 8 of 35 prostatitis patients, 23%, exhibited inflammation in their EPS at the first prostate massage (>=10 WBCs/HPF). At the fourth tri-weekly massage, that number rose to 22 of 35 patients, or 71% . If four successive massages are required to expel inflammatory markers from the prostate in these cases, we cannot assume that bacteria, if they are present, will appear in significant numbers with fewer massages. Therefore, a single massage is not a valid test of the presence of bacteria in the prostate.
This problem gets even worse if the EPS is diluted by urine (VB3). In a sample of 463 men with CP/CPPS, Nickel el al[13] found that of the patients whose EPS met the >=10 WBCs/HPF threshold, only 44% of these met the same threshold using VB3. Likewise, in 49 patients whose EPS met this threshold, Wheeler[14] found that only 39% qualified using VB3. Yet, practice guides state that EPS and VB3 can be used interchangeably for diagnosis.[15],[16]
A study by Guercini et al[17] examined 56 prostatitis patients not
affected by bacteriuria and therefore
classified as having non-bacterial prostatitis, according to the prevailing NIH
standard. “Using the transperineal route, ultrasound guided needle aspirates
were taken from sonographically dishomogenous areas in the prostate … Cultures
were positive for aerobic (56%) and anaerobic (23%) agents.” This sampling method avoided possible
contamination from either the urethra or the rectum and did not depend on
performing sufficient massages. A total of 72% of prostatitis patients traditionally
classified as "non-bacterial" were found to have bacteria in their
prostates.
Kreiger[18]
selected 135 men with refractory prostatitis and no evidence of bacteria by
traditional methods. Using
So when investigators look carefully
for bacteria, over 70% of prostatitis patients regarded as having non-bacterial
prostatitis are found to have bacteria in their prostates. The alone does not
prove the bacteria are the cause of the prostatitis. We will get to that in due
course.
The CP/CPPS diagnostic category is overly broad, lumping chronic prostatitis together with other issues. Differentiation among different pathologies is needed. To this end, a system called "UPOINT" has been created. UPOINT distinguishes among six different prostate and pelvic dysfunctions, and treats each accordingly. According to a recent UPOINT study[19], the proportion classified as "infection" was very low: 13% ureaplasma and only 2% other bacteria. There is no indication that more than one massage was used to obtain EPS. In addition, all patients with NIH prostatitis type II were excluded - prostatitis with recurrent UTI. So composition of the sample was biased against patients with bacterial prostatitis.
Moreover, any gram positive bacteria other than Enterococcus, such as Staphylococcus, were regarded as not causing infection. Weidner does not agree with this view. Referring to Staphylococcus, Weidner says: "According to our data only a few patients have a typical 'prostatitis' pattern of gram-positive bacteria and a concomitant leukocyte reaction; nevertheless, we believe that these bacteria are important etiologic agents in some cases of prostatitis."7 So the Weidner study suffered from a low rate of detection due to single massage, rather than disregard of a potential pathogen.
In the UPOINT study 64% of patients were reported to have "organ specific" pathology, which included leukocytosis. So any patient with copious leukocytes found in association with gram positive bacteria would be regarded as "organ specific" rather than infection. If we add the cases of "organ specific" to the number of acknowledged infections, the result is 79% of patients, in accord with the other data.
Of course, even if a large percentage of prostatitis patients have bacteria and inflammation in their prostates, this alone does not prove that those bacteria are causing the prostatitis. To prove this, we must eradicate the bacteria and observe relief of the prostatitis symptoms.
Argument 2:
Antibiotics don’t help
A 2008 study by Nickel and Xiang[20] considered 261 men with recent onset prostatitis, median duration 3.5 weeks. These patients were not restricted to the NIH type II/ repetitive UTI classification[21]. They compared antibiotic cure rates for bacteria traditionally recognized as prostate pathogens with “non-traditional” prostate bacteria. In both cases they found a close correlation between microbiological cures and clinical cures, with cure rates ranging from 70-78% of patients. Among the bacteria involved were 25 different species of Staphylococcus and Streptococcus. Of the patients who were initially cured, over 77% had achieved a durable cure at 6 month follow-up, both clinically and microbiologically. So promptly applied antibiotics are effective in a large majority of cases.
Yet an earlier study by
Nickel[22]
is widely cited as evidence that antibiotics don't work. The study population were men with long-standing prostatitis, 80% of
whom had already failed treatment with antibiotics. Now let us consider how
this study sample came to be: the pretreated patients were originally part of
an earlier population treated with antibiotics, some of whom were cured. The
patients who were cured moved on, and only the failures were retreated. So this
is a biased sample. All this study tells
us is that patients who have already
failed antibiotic therapy once are likely to fail again.
Nonetheless, we need to inquire why antibiotics do not work in such cases.
Bacteria
that are able to form a sessile colony secrete a biofilm which impedes the
action of leukocytes. However, nutrients and antibiotics can circulate freely
within. Absent a biofilm, destroying the vast majority of bacteria is usually
sufficient for leukocytes to finish the job. When protected by a biofilm, if a
single resistant bacterium survives antibiotic assault, it will repopulate the
biofilm with resistant bacteria. In addition, some biofilm bacteria become
dormant "persister" cells and are unaffected by the normal physiological
concentration of antibiotics. Persister cells will resume normal metabolism
only when the bacterial population density is reduced and conditions
become favorable. So when antibiotics are withdrawn the
bacteria repopulate the biofilm.[23]
Biofilms readily form in urinary catheters and play a role in the development of infected urinary tract calculi.[24] Biofilms can form within any body cavity, such as the prostatic acini and ducts: "Specific electron micrographs demonstrate exopolysaccharide coated microcolonies of bacteria firmly attached to the ductal and acinar walls."[25]
Biofilms attach to prostatic
calcifications. Of 150 bacterial strains obtained
from prostatitis patients, Mazzoli[26]
found 85% were strong or medium biofilm producers. Mazzoli arranged to collect
prostate calcifications in a sterile manner from five patients who were
undergoing TURP. All five displayed biofilms and contained viable bacteria.
Zhao et al[27] compared the efficacy of antibiotic treatment in patients with and without calcifications. They found similar rates of microbiologic cure immediately after treatment, but a high rate of relapse among the patients with calcifications, at follow up 3-8 months later. Differences between groups at follow-up were significant at the p<.01 level:
|
|
|
% Microbiological cure |
Median NIH-CPSI score |
||
|
Patients: |
N |
End of treatment |
3-8 Months after |
Before treatment |
3-8 Months after |
|
With calculi |
39 |
82.1 |
43.6 |
24 |
19 |
|
Without calculi |
62 |
87.1 |
72.6 |
24 |
11 |
Table 1: Effect of
calcifications on relapse after antibiotic therapy, per Zhao et
al28
"There was a noticeable decrease in the cure rate of CBP patients with prostatic calculi due to relapse after antimicrobial therapy."
A wide variety of bacteria can be prostate pathogens, and
given time are likely to form treatment resistant biofilms. The key to preventing this adverse outcome
is prompt antibiotic treatment when symptoms arise.
For long-standing cases of prostatitis, direct injection of antibiotics into the prostate offers hope of cure.
Guercini[28] recruited 320 patients of average prostatitis duration 5 years, who had failed repeated cycles of antibiotics. High concentrations of antibiotics were injected directly into the prostate three times. At 6-month follow-up 68% of patients reported marked improvement or complete resolution of symptoms. This paper was not peer reviewed and skepticism of these results has been voiced.[29]
More recently, Min et al[30] [31]recruited
283 patients, course of disease 1~22
years. They performed a 3-arm study,
divided among the following treatments:
1.
A
Chinese "patent medicine"
2.
Oral
antibiotics chosen per sensitivity of prostate bacteria
3.
Direct
injection of antibiotics into the prostate, plus oral antibiotics, plus
instillation of antibiotics into the urethra, chosen per sensitivity of
prostate bacteria.
Here are their results:
|
Treatment arm |
n |
NIH-CPSI score before treat-ment |
std dev |
NIH-CPSI score 8 weeks after start of treatment |
std dev |
Improve-
ment in NIH-CPSI score |
Normal EPS 30 days after start of treatment, % |
Microbio- logic cure 30 days after start of
treatment, % |
Normal EPS 3 months after start of treat-ment, % |
|
Patent medicine |
94 |
16.3 |
5.6 |
13.2 |
3.2 |
3.1 |
32.2 |
23.9 |
31.9 |
|
Oral antibiotics |
94 |
15.3 |
5.6 |
10.2 |
2.2 |
5.1 |
51.0 |
68.0 |
44.6 |
|
Multiroute antibiotics |
95 |
19.4 |
5.2 |
5.2 |
3.2 |
14.2 |
79.0 |
89.5 |
84.2 |
Table 2: Results of multi-route administration of antibiotics into the prostate per Min et al.
We conclude that over 70% of prostatitis is bacterial in origin and that promptly applied antibiotics will cure most of these cases of prostatitis. In the remainder of this paper we dispose of the remaining arguments against bacterial prostatitis.
Argument 3: “Every
man has bacteria in his prostate”
One reason prostate bacteria have been deemed irrelevant is that many, perhaps all, asymptomatic men have bacteria in their prostates. Let us consider bacteria elsewhere in the body. About 50% of humanity harbor helicobacter pylori.[32] Yet the prevalence of peptic ulcer disease is only 5.6%, lifetime, in one study[33] and 4.1% in another.[34] A Chinese study found a 73% prevalence of H. pylori but only 17% of peptic ulcer.[35] So an infected individual has only an 8-23% chance of developing disease.
Regarding tuberculosis, "Nearly one-third of world population is latently infected with M. tuberculosis", yet only "5%-10% of infected individuals will develop active disease during their life time."[36]
It would be unthinkable to not treat active tuberculosis or peptic ulcer, just because the vast majority of carriers are asymptomatic. Even if 100% of men were to have known pathogenic bacteria in their prostates, a prevalence of 6-13% of prostatitis would be similar to the rate of illness caused by M. tuberculosis. So a wide prevalence of asymptomatic carriers of a species of bacteria does not exonerate that bacteria from being a dangerous pathogen. In particular, as shown above, bacteria that some have regarded as benign, such as staphylococcus, can cause prostatitis.
Argument 4:
Asymptomatic patients also have inflammation of the prostate
If one set of patients with prostate inflammation do not experience symptoms, how can inflammation cause pain in other patients?
Fehri et al[37] studied asymptomatic prostates that were removed due to cancer. They found Propionibacterium acnes bacteria in 58 out of 71 (81.7%) prostate tissue samples, "but was absent from healthy prostate tissues (20 samples) and other cancerous tissue biopsies (59 mamma carcinoma samples)." Why should P. acnes infection generally be asymptomatic? The answer is provided by visualization studies performed by Alexeyev, et al.[38] From these studies it can be seen that P. acnes is an intracellular parasite that forms numerous, dispersed, intracellular colonies spanning only a few host cells at a time. Although its presence is inflammatory at the cellular level, this inflammation is apparently too diffuse to cause pain. Thus P. acnes infections may continue indefinitely without prompting treatment.
P. acnes is not necessarily the only cause of asymptomatic inflammatory prostatitis. Korrovits et al[39] studied seminal fluid as a means to assess asymptomatic prostate inflammation. Seminal fluid of all subjects was found to contain some bacteria; however, subjects with inflammatory markers typically had about eight times more bacteria than controls. Coryneform bacteria were found significantly more often in these patients, with 81% harboring such bacteria vs. only 38% of controls.
So it would seem that the bacteria typically implicated in asymptomatic inflammatory prostatitis are different from those typically found in symptomatic patients.
Asymptomatic prostatitis is widespread. Wu et al[40], found asymptomatic prostatitis in 21% of Chinese men. Korrovits found the prevalence to be as high as 19%.
Chronic inflammation is a known carcinogen: "Helicobacter pylori infection of the stomach ... is accompanied by inflammation, gastric atrophy and subsequent gastric carcinoma."[41] In symptomatic prostatitis: "Men with a history of prostatitis were more likely to self-report a history of prostate cancer (26% versus 7%; P < 0.0001)."[42] Men whose prostate inflammation was detected on biopsy had 1.78 times the risk of subsequently developing prostate cancer compared to men with no inflammation detected. Odds ratio increased to 2.24 for high grade disease, Gleason score 7-10.[43]
Symptomatic or not, chronic prostate inflammation elevates the risk of prostate cancer. Prostate cancer is the second leading cause of male cancer deaths, and is the most common malignancy in older men in the Western world. So the chronic inflammation of asymptomatic prostatitis is not a benign condition. It merits treatment just as does the symptomatic variety.
Argument 5:
Antibiotics are Anti-Inflammatory
In this context "anti-inflammatory" refers to the theory that antibiotics are intrinsically anti-inflammatory apart from their anti-bacterial properties. This idea likely got its start with the observation that prostatitis sufferers relapsed after the cessation of antibiotic treatment. However, as we have seen above, this is likely due to biofilms repopulating rather than some intrinsic anti-inflammatory effect.
We will focus on fluoroquinolones: "Because of their broad spectrum activity and preferential accumulation in prostatic fluid, fluoroquinolones have become the standard of care for chronic bacterial prostatitis."[44]
Yasumoto et al[45] [46] tested the effect of sparfloxacin on 17 men considered to have non-bacterial prostatitis. In ten of these patients, elevated cytokines were reduced to undetectable levels after treatment. The cytokine status of the remaining seven was not reported. Treatment was at least somewhat effective in reducing symptoms in 76% of the men. Calcifications were noted in 29%. The investigators do not state to what extent this subgroup benefited from treatment.
The men were determined to have non-bacterial prostatitis by the absence of "Gram-negative bacteria number of 10^3/ml or more in a culture of the semen and VB3 urine " They ignored all gram positive bacteria, including known prostatitis pathogen Enterococcus faecalis, and did not attempt to detect some known Gram-negative pathogens: "there is a possibility that gram negative bacteria such as Chlamydia trachomatis and Ureaplasma urealyticum are involved in such cases ... an adequate search for these microorganisms was not carried out". This study failed to exclude bacterial prostatitis! It does not qualify as evidence of an "anti-inflammatory" effect. Nevertheless, it has been cited 22 times.
Dalhoff reviewed the published data on fluoroquinolones["FQs"].[47] He included the Yasumoto study uncritically, and proceeded to opine that FQs generally "attenuate cytokine responses." He did find decreases in IL-1 and TNF. The reduction of TNF reduced sepsis-induced mortality in animal models.
However, there were important pro-inflammatory effects: "The marked activity of sparfloxacin and moxifloxacin against Listeria is due to ... a quinolone mediated increase in IFN-γ levels." Also, "FQs were found to upregulate hematopoiesis," the result being: "... ciprofloxacin and moxifloxacin significantly enhanced GM-CSF production in the lungs of immunocompromised animals (3.5- to 4-fold) ... GM-CSF has a pivotal role in establishing and maintaining resistance to local infections ... several clinical studies indicate that ciprofloxacin may shorten the duration of chemotherapy-induced neutropenia."
More recently Shiratori et al[48] found: "... quinolones significantly enhanced OPN [Osteopontin] secretion". Osteopontin is an upregulator of chemotaxis. "Within the immune system, OPN is a cytokine secreted by activated T cells, NK cells, dendritic cells, and macrophages ... the expression of OPN correlated with an effective immune and inflammatory response."[49]
A recent study of fluoroquinolones in patients with complicated UTIs showed they were pro-inflammatory: "... ciprofloxacin and levofloxacin induce more reactive oxygen species."[50]
In sum, fluoroquinolones exhibit numerous
pro-inflammatory effects and a few anti-inflammatory effects. We cannot characterize them as
"anti-inflammatory".
Argument 6: Muscle
Tension
Some patients have pelvic pain due to muscle tension. Here is an account from a patient who was completely relieved of his pelvic symptoms by meditation: "... while I ate (too fast) I tensed my forehead, while I talked I tensed my shoulders, while I listened I tensed my neck, while I drove I tensed everything. ... My spine was hunched rigid. My stomach turned to rock. And yes, my pelvic floor was hoisted up tight."[51]
In a study entitled: "Usefulness of a Myofascial Trigger Point Injection for Groin Pain in Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Pilot Study", the authors report the NIH-CPSI score improved from 20.2 to 12.5, P<.05.[52] They state: "In patients with myofascial pain syndrome ("MPS"), palpation of the affected muscles elicits pain, typically the pain that patients attribute to their 'prostatitis.' ” The men selected for their study all had MPS of the iliopsoas. Nineteen of the 21 had known musculoskeletal problems, including injury affecting the groin or a herniated lumbar disk. It is not clear that any had prostatitis.
The originator of the Wise-Anderson/Stanford protocol reports a complete cure using his methods of paradoxical relaxation and trigger point release.[53] In a recent study, Anderson et al reported the median NIH-CPSI total score of participants was 26 before treatment, and 19 after[54], a 7-point improvement. In contrast, we have already seen that the NIH-CPSI score of patients with early onset prostatitis, when promptly treated with antibiotics, improves from 24 to 11,[20] a 13 point improvement. A study of the effect of removing the prostate for severely symptomatic prostatitis patients, the median CPSI score dropped from 35 to 10 after 6 months, an improvement of 25 points (p=.03).[55] If the problem were muscle tension these treatments would have made no difference.
In the
Conclusion:
Over 70% of prostatitis is caused by bacteria. A wide variety of bacteria can be prostate pathogens. The large majority of these cases are curable via prompt application of antibiotics. Long standing cases may be salvageable by intensive antibiotic therapy including direct injection of antibiotics into the prostate.
The aggregation of pelvic muscle tension with prostatitis under the CP/CPPS banner is inappropriate. Differential diagnosis is required. These ailments must be treated separately and appropriately, both in research and at the clinic.
The dominance of the bacterial etiology of prostatitis
appears to be well known to researchers in
A major revision is needed in the attitude of our medical profession towards prostatitis. The prostate must be monitored regularly and pro-actively for signs of prostatitis, and treated promptly and aggressively with antibiotics when it arises. Failure to do so condemns a large number of prostatitis sufferers to permanently impaired quality of life, significantly increased risk of prostate cancer, and endless haunting of urologists' offices, fruitlessly seeking a cure.
References: